
Peripheral nerve stimulation (PNS) has broad applications for pain relief and has been successfully used to treat myriad types of pain. Current conventional neurostimulation systems for pain relief typically involve a brief (e.g., ≤7-10-day) trial to assess presumed effectiveness prior to permanent system implantation. Conventional trials are typically considered to be positive if patients report a certain threshold of pain relief (e.g., ≥50% pain relief), and positive short trials are then often followed by implantation of a permanent neurostimulator. While patients may achieve successful long-term pain relief from a permanently implanted system, many experience inadequate pain relief resulting in system failure and explant. Overall, low trial-to-implant conversion rates and high rates of explant due to inadequate pain relief suggest that improved real-world patient identification strategies are needed to distinguish likely responders and non-responders to neurostimulation such that, if sustained relief is not achieved from a short-term PNS treatment, patients and physicians are better informed before making the decision to implant a permanent system.
In a recently published manuscript in the Journal of Pain Research entitled, “60-Day PNS Treatment May Improve Identification of Delayed Responders and Delayed Non-Responders to Neurostimulation for Pain Relief”, Dr. Ramana Naidu and colleagues — Drs. Sean Li, Mehul Desai and Samir Sheth along with members of SPR Therapeutics’ R&D team, Nathan Crosby, PhD, and Joseph Boggs, PhD — explored how SPRINT PNS, a 60-day temporary PNS system, could be leveraged to help physicians characterize patient responsiveness to neurostimulation. The study represented the first analysis of real-world evidence showing the evolution of pain relief in patients during a temporary, 60-day PNS treatment for pain relief.
By evaluating patient reports of pain relief throughout the course of the 60-day PNS treatment period, the study authors found that patients could be categorized into four response profiles:
- Early Responders: patients who reported ≥50% pain relief throughout the 60-day treatment period;
- Early Non-responders: patients who never achieved ≥50% pain relief during the 60-day treatment period;
- Delayed Responders: patients who reported less than 50% pain relief within the first two weeks but ultimately achieved ≥50% pain relief before the end of the 60-day treatment period; and
- Delayed Non-responders: patients who reported ≥50% pain relief within the first two weeks but ultimately fell below 50% pain relief by the end of the 60-day treatment period.
Early responders and early non-responders displayed patterns within the first two weeks of stimulation that were highly indicative of their final disposition at the end of the 60-day treatment. Meanwhile, delayed responders and delayed non-responders, who represented nearly 40% of all patients, evolved over the course of the 60-day treatment such that their early responses in the first two weeks did not reliably predict whether the treatment would ultimately be considered successful after up to 60 days.
The potential clinical benefits of improving patient identification strategies are significant. In the case of delayed responders, clinical and real-world evidence suggest that responders to 60-day PNS treatment have the potential to achieve sustained benefits that may long outlast the 60-day treatment period, obviating the need for a permanently implanted conventional neurostimulation system in many cases. In patients for whom pain returns soon after the end of the 60-day treatment, success during the temporary 60-day PNS treatment could also validate trialing a permanent implant. Therefore, whereas delayed responders undergoing a brief (≤7-10-day) temporary stimulation period may be spuriously categorized as non-responders and be forced to seek alternate therapeutic options, a 60-day treatment period may increase patient access to PNS by either providing sustained pain relief or providing evidence for the potential utility of a permanent implant. Conversely, the potential costs and risks are significant for delayed non-responders who initially present strong false positive responses during the brief trial period, and on that basis are qualified for permanent implantation that is ultimately unlikely to be successful. Identifying delayed non-responders more effectively, potentially with a longer (i.e., 60-day) treatment period, could therefore help avoid the physical, psychological and financial impacts associated with implantation, revision, and explant of neurostimulator systems in non-responsive patients.
These concepts are illustrated in the figure below, showing the potential outcomes of positive and negative trials with conventional PNS systems, as well as the potential improvements in patient identification enabled by a longer, 60-day treatment period. These scenarios support the importance of an extended 60-day temporary peripheral nerve stimulation period to help inform stepwise treatment strategies that may optimize outcomes and cost-effectiveness.
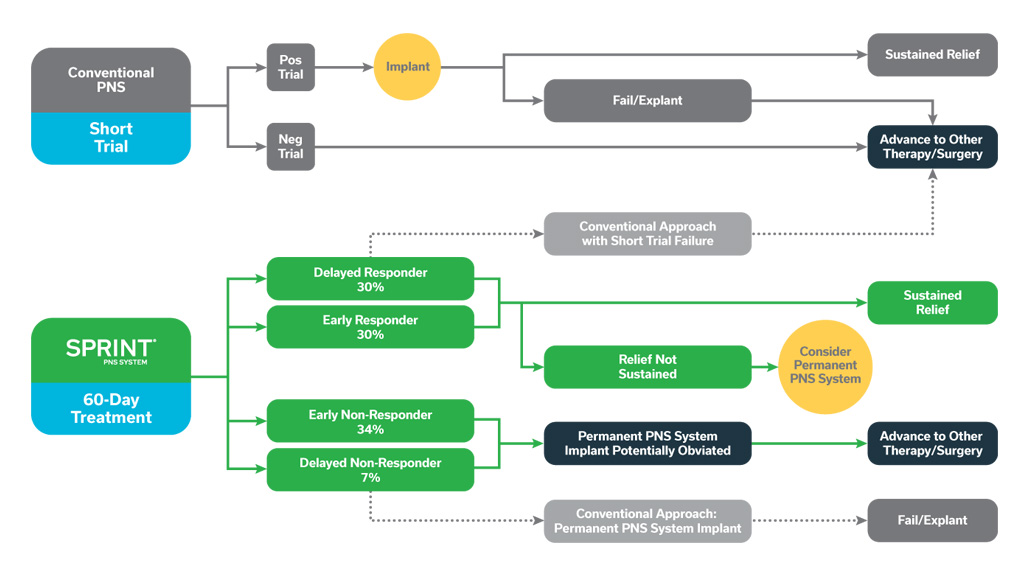
The potential outcomes of positive and negative conventional, brief PNS trials are shown above, with many patients proceeding to permanent implant but ultimately failing to achieve adequate pain relief and requiring system explant. A temporary, 60-day PNS treatment may improve identification of responders, providing some with sustained relief, while either obviating or validating the potential utility of permanently implanted PNS in others.
Click to read the full manuscript in the Journal of Pain Research, Volume 15
The SPRINT PNS System is indicated for up to 60 days for: (i) Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain; (ii) Symptomatic relief of post-traumatic pain; and (iii) Symptomatic relief of post-operative pain. The SPRINT PNS System is not intended to be placed in the region innervated by the cranial and facial nerves.
Physicians should use their best judgment when deciding when to use the SPRINT PNS System. For more information see the SPRINT PNS System IFU. Most common adverse events are skin irritation and erythema. Results may vary. Rx only.
Important safety & risk information: https://bit.ly/2FU92NH

 The PNS Trifecta: All Three SPRINT® PNS System Abstracts Submitted to ASRA 2021 Pain Medicine Meeting Receive President’s Choice Award
The PNS Trifecta: All Three SPRINT® PNS System Abstracts Submitted to ASRA 2021 Pain Medicine Meeting Receive President’s Choice Award

Leave a Reply