
Cleveland, Ohio – May 4, 2023 – SelectHealth, a non-profit health plan with over one million members, has demonstrated leadership amid the opioid crisis by creating one of the first coverage policies supporting the use of Peripheral Nerve Stimulation (PNS). SPR Therapeutics is pleased to see this occur at a time when PNS, such as that delivered by the SPRINT® PNS System, is fast becoming a leading and mainstream non-opioid treatment for chronic pain. With more than thirty peer-reviewed publications supporting the use of SPRINT PNS across a wide range of pain conditions, SelectHealth is taking steps to assure access for their members to this FDA-cleared treatment as well as other PNS treatments.
“Chronic pain is a major driver of healthcare utilization. Chronic shoulder and knee pain collectively represent the largest group of patients with peripheral pain being treated using the SPRINT PNS System. Major limitations on day-to-day living are often seen in patients dealing with shoulder and knee pain. Fostering patient access to an effective non-opioid option is an essential step in restoring quality of life to this large group of pain sufferers,” said Dr. Marc Huntoon, Director of Medical Affairs at SPR Therapeutics. “SelectHealth is demonstrating its commitment to their members by expanding access to a type of treatment that can help patients avoid the ongoing use of opioids, the need for surgery, and permanently implanted devices. As we have studied real-world outcomes on the relief of pain and improvements in quality of life experienced by more than 6,000 SPRINT PNS patients, I trust that other payers will soon follow suit and foster coverage of PNS for their members.”
“We applaud SelectHealth for their leadership in allowing members access to evidence-based, non-opioid treatment options such as SPRINT PNS,” said Mark Stultz, SPR Senior Vice President, Market Development. “We are collaborating actively with members of Congress, medical societies, physician researchers, health economists and payers to advance evidence that supports the responsible clinical application of SPRINT to patients in need of a solution to address their pain. We have invested heavily in this work and in our SPRcare patient access program to help patients gain payer approval for coverage of the PNS treatment in cases where it is medically necessary and prescribed by their pain management physician and to do so in a shorter period of time.”
About the SPRINT PNS System
The SPRINT® PNS System, by SPR® Therapeutics, marks an innovative shift in the treatment of pain. Our breakthrough, 60-day treatment is a First-Line™ PNS option uniquely proposed to recondition the central nervous system to provide significant and sustained relief from chronic pain — without a permanent implant, nerve destruction or the risk of addiction. The system has been studied extensively for low back pain, shoulder pain, post-amputation pain, and chronic and acute post-operative pain, is cleared for use up to 60 days, and is recognized by leading pain management centers. Market research indicates that this breakthrough neuromodulation treatment is a patient-preferred alternative to more invasive options.
The SPRINT PNS System is indicated for up to 60 days for: Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain; symptomatic relief of post-traumatic pain; symptomatic relief of post-operative pain. The SPRINT PNS System is not intended to be placed in the region innervated by the cranial and facial nerves.
Physicians should use their best judgment when deciding when to use the SPRINT PNS System. For more information see the SPRINT PNS System IFU. Most common adverse events are skin irritation and erythema. Results may vary. Rx only.
For additional information regarding safety and efficacy, visit: SPR Safety Information.
About SPR Therapeutics, Inc.
SPR Therapeutics is a privately held medical device company, providing patients with a non-opioid, minimally invasive pain treatment option. Our SPRINT® PNS System fulfills a critical unmet need for a drug-free, surgery-free option for millions who suffer from chronic pain. Backed by the largest body of clinical evidence in peripheral nerve stimulation for the treatment of pain, SPR has demonstrated commercial demand in untapped peripheral (shoulder and knee) and back pain markets and built an incredibly strong foundation for commercial growth. Headquartered in Cleveland, OH with satellite offices in Chapel Hill, NC and Minneapolis, MN, SPR’s Senior Management team includes experienced industry veterans with nearly 200 years of collective pain market and MedTech expertise, all driven by our purpose – to improve the quality of patients’ lives by providing them with a minimally-invasive, drug-free, surgery-free solution to manage their acute and chronic pain.
More information can be found at www.SPRTherapeutics.com.
SPR Contacts:
Michelle McDonald
Vice President – Marketing
844.378.9108
Dave Folkens
Public Relations
612.978.6547

 New SPRINT extensa XT® System with Bimodal PNS™ Offers Synergistic Treatment of Pain
New SPRINT extensa XT® System with Bimodal PNS™ Offers Synergistic Treatment of Pain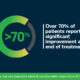

Leave a Reply