Real-world evidence (RWE), derived from analysis of data from the routine use of a treatment or therapy in general clinical practice, has an important role in providing insight into treatment effectiveness outside of the environment of clinical research. Increased recognition of the importance of RWE has led to recent calls in the neuromodulation field to increase the collection and sharing of RWE to improve clinical practice and clinical guidelines. The SPRINT PNS® System, which delivers short-term peripheral nerve stimulation percutaneously for up to 60 days, has been studied extensively in clinical trials, demonstrating significant and sustained chronic pain relief in a majority of patients across multiple pain indications such as chronic shoulder pain, low back pain, post-operative pain and post-amputation pain. However, outside of small retrospective case series and individual case reports, few real-world data have been published regarding the use of 60-day PNS.
Dr. Matthew Pingree and colleagues – Drs. Mark Hurdle, David Spinner and Ali Valimahomed along with members of SPR Therapeutics’ R&D team, Nathan Crosby, PhD and Joseph Boggs, PhD – recently published a manuscript in Pain Management representing the largest set of real-world data to date regarding the effectiveness and long-term impact of 60-day PNS treatment. The team of investigators sought to determine if 60-day PNS treatment could provide long-term relief in a real-world setting based on the results of a cross-sectional follow-up survey of patients who previously underwent treatment with the SPRINT PNS System.
The survey was distributed to patients from a national real-world database of SPRINT PNS users who had previously agreed to opt into a follow-up survey. It used validated pain outcomes to assess patients’ current pain levels, changes in quality of life, and changes in analgesic medication usage compared to before they started the SPRINT treatment. Analysis focused on the 252 survey respondents who were at least three months from the time they started PNS treatment, and follow-up durations ranged up to 30 months.
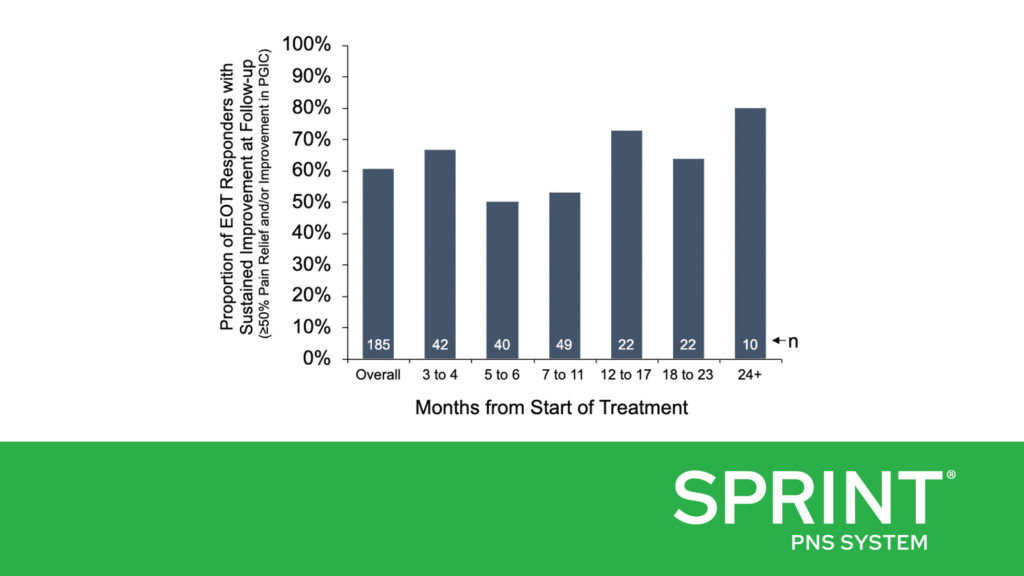
Dr. Pingree and colleagues found that 73% of the survey respondents who were at least three months from the time they started PNS treatment (185/252) had been treatment responders at the end of the 60-day treatment, with at least 50% pain relief and/or clinically significant improvement in their quality of life. At the time of survey completion, 61% (112/185) of those treatment responders reported sustained improvements in pain and/or quality of life, including some of those who were more than two years post-treatment (Figure A). This RWE corroborates previously published clinical studies with follow-up durations of up to 12 months, which have consistently found that a majority of subjects reported substantial pain relief (≥50%) and resulting improvements in function. The RWE also supports that many patients can achieve sustained long-term improvements even outside the more rigorous environment of a prospective clinical trial and indicates that the outcomes of previously published prospective studies can be replicated in broader clinical practice.
Additionally, 35% (44/126) of those that reported using opioids before beginning the 60-day PNS treatment and 32% (47/147) of patients reporting anti-convulsant (e.g., gabapentin, pregabalin) usage before beginning treatment reported reducing or ceasing usage as of the time of survey completion (Figure B). Reducing analgesic medication usage, especially opioid analgesics, has become a priority of physicians in recent years in response to the opioid epidemic. Reducing chronic analgesic usage can also help reduce the substantial socioeconomic costs and burden on the healthcare system of opioid abuse and misuse. As a nonpharmacological intervention that does not require permanent implantation and may help reduce reliance on opioid and non-opioid analgesics, 60-day PNS can be an important and minimally invasive part of the treatment options available to physicians and patients for pain relief.
While the survey did not contain questions about adverse events as the patients had already completed treatment, data collected by SPR show that the most commonly reported adverse event has been adhesive-related skin irritation. Serious adverse events have occurred in less than 1% of patients.
This study represents the largest body of real-world evidence published to-date supporting the effectiveness of 60-day PNS treatment, including significant and sustained pain relief and improvements in quality of life for a large proportion of respondents. These real-world data are consistent with the findings of published prospective studies that have demonstrated significant and sustained improvements following short-term PNS and support the benefits of 60-day PNS for the treatment of a wide range of pain conditions in broader clinical practice.
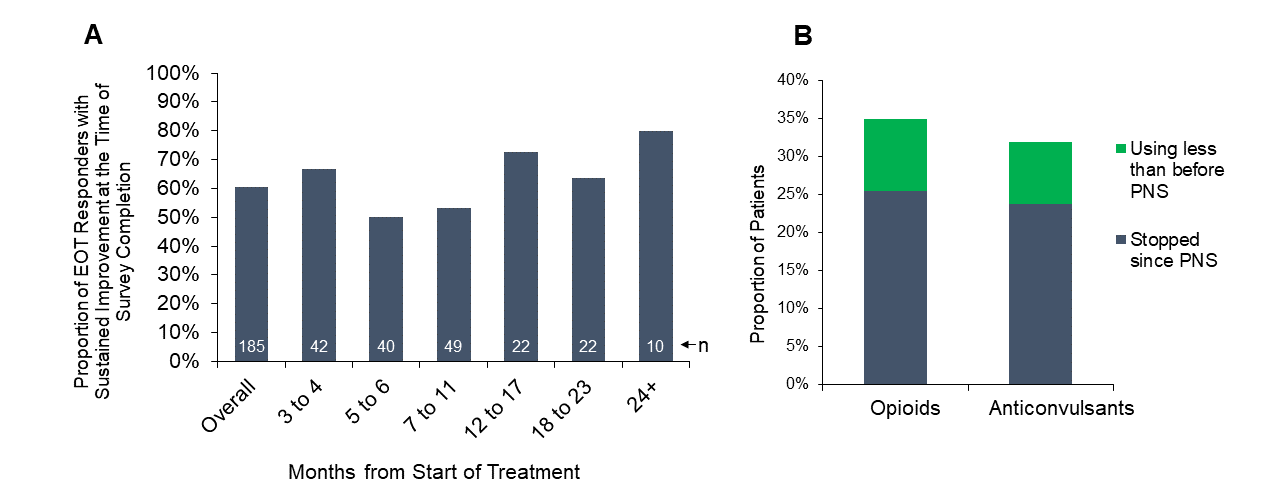
Figure A) Proportion of patients who were responders at the end of treatment (EOT Responders) and maintained at least 50% pain relief and/or improvement in quality of life at the time of survey completion, grouped by months from the start of treatment. Figure B) Proportion of patients who were using opioid medications (n=126) or anti-convulsant medications (n=147) before beginning treatment who reported cessation or reduction of opioid or anti-convulsant medications at the time of survey completion.
The SPRINT PNS System is indicated for up to 60 days for: (i) Symptomatic relief of chronic, intractable pain, post-surgical and post-traumatic acute pain; (ii) Symptomatic relief of post-traumatic pain; and (iii) Symptomatic relief of post-operative pain. The SPRINT PNS System is not intended to be placed in the region innervated by the cranial and facial nerves.
Physicians should use their best judgment when deciding when to use the SPRINT PNS System. For more information see the SPRINT PNS System IFU. Most common adverse events are skin irritation and erythema. Results may vary. Rx only.
Important safety & risk information: https://bit.ly/2FU92NH

 SPR Therapeutics Announces 10,000th SPRINT PNS Procedure for Patients Suffering from Life-Altering Pain
SPR Therapeutics Announces 10,000th SPRINT PNS Procedure for Patients Suffering from Life-Altering Pain 

Leave a Reply