MRI safety information
NOTE: Patients that received a SPRINT System prior to September 1st, 2022, should contact SPR Therapeutics to obtain specific MRI safety information. (Phone: 844-378-9108)
All other SPRINT patients should use the information listed below:
The SPRINT PNS System is MR Unsafe. – All MRI procedures, no matter the anatomic site, are contraindicated for patients with SPRINT PNS System. Exposure can cause tissue heating and injury or unwanted stimulation. Pull out the Lead and remove all other System components from the patient before an MRI examination is performed. In the case of a Lead fracture beneath the skin resulting in a retained Lead remnant, an MRI examination is safe to perform under the conditions described in the MR Conditional statement below.

A retained Lead Remnant ONLY is MR Conditional. – A person with a retained Lead remnant may be safely scanned anywhere in the body at 1.5T or 3.0T under the following conditions. Failure to follow these conditions may result in injury.

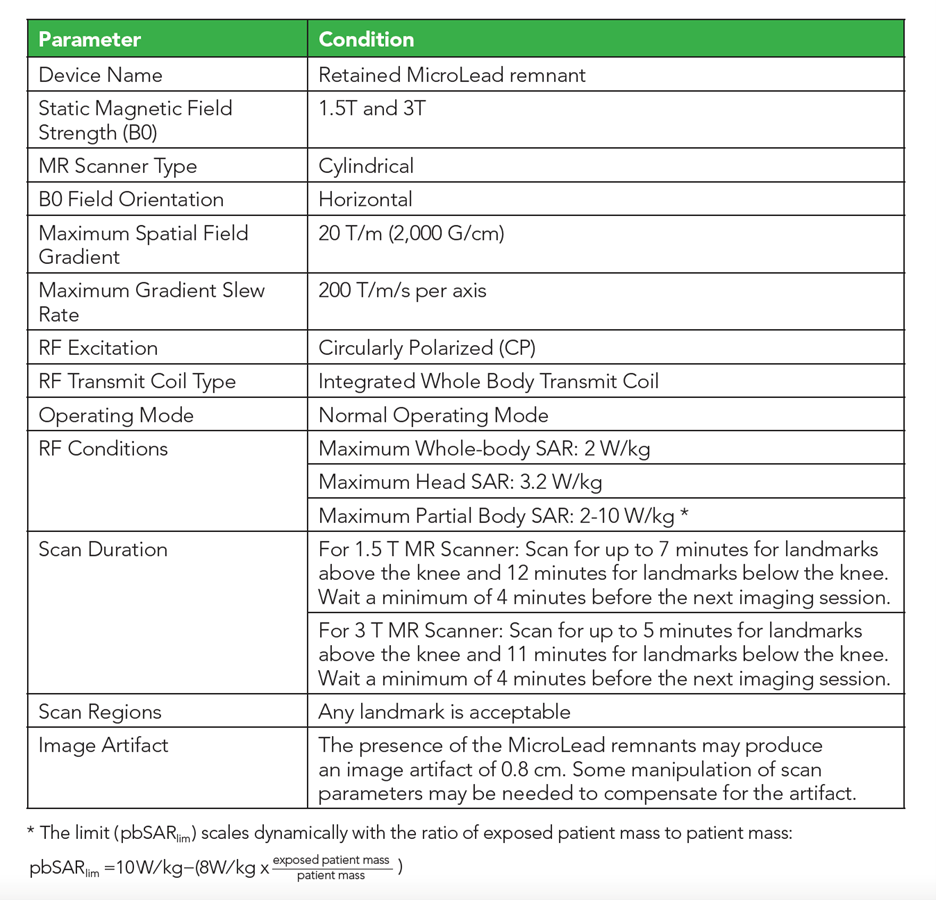
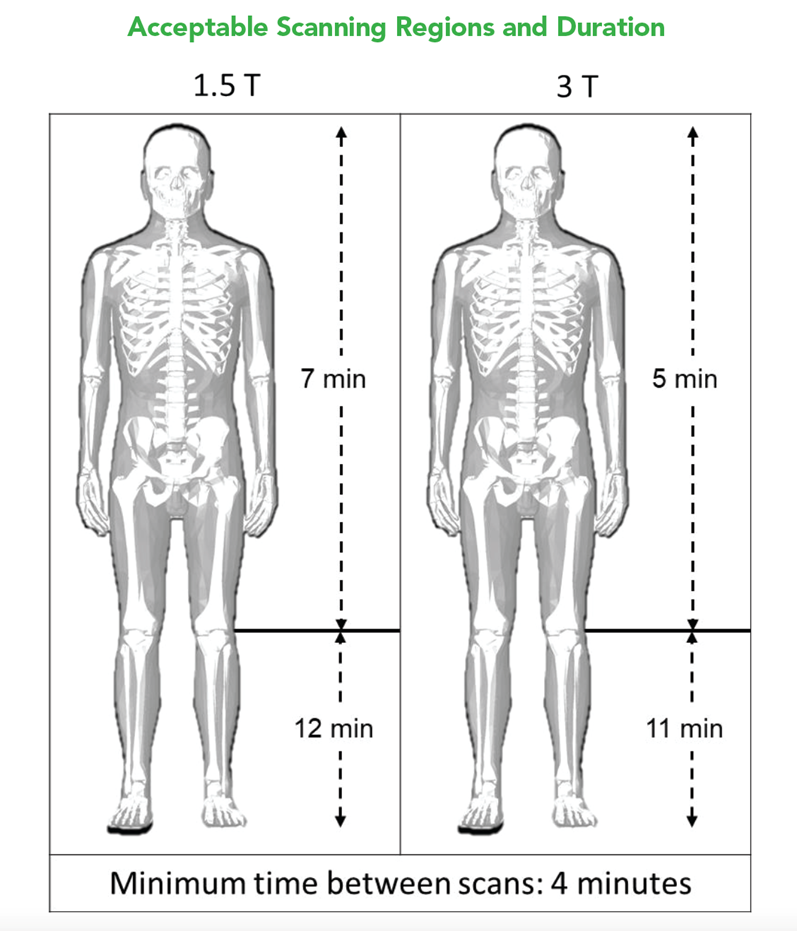
NOTE: There should be no section of Lead visible above the skin.
NOTE: If a Lead is placed in an individual with an existing retained Lead remnant, the new Lead should not be placed in a location where it could touch the original Lead remnant. During MRI, two MicroLead fragments touching can result in an increase in temperature of the Lead remnants and surrounding tissue where they touch.

